
|

|
THIRD ASIA PACIFIC
PHARMACEUTICAL
COMPLIANCE CONGRESS
AND BEST PRACTICES
FORUM
|
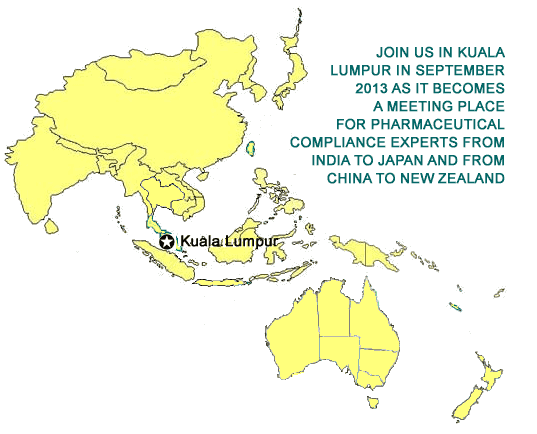
ATTEND ONSITE
September 10 - 12, 2013
PARKROYAL Kuala Lumpur Hotel
Kuala Lumpur, Malaysia
|
WEBCAST PARTICIPATION
In your own office or home live via the Internet with 24/7 access for six months
|

|
KEYNOTE SPEAKERS
|

Ian Bell
President, Allergan Asia Pacific, Singapore |

Dominique Laymand, Esq.
Vice President Compliance & Ethics EMEA, (Europe, Middle-East, Africa, Russia and Turkey), Bristol-Myers Squibb, President, International Society of Healthcare, Ethics and Compliance Professionals (ethics), Paris, France |

Dr. Jean-François Manzoni, MBA, PhD
Shell Chaired Professor of Human Resources and Organisational Development; Director, INSEAD Global Leadership Centre (IGLC), Professor of Management Practice, INSEAD, Singapore
|

Prof. Dr. Agus Purwadianto, MD, JD, PhD
Senior Staff on Legal & Human Rights Affairs, Coordinating Ministry for People's Welfare, Indonesia, Member, Commission for Bioethics, Member, Medical Ethics Board, Indonesian Doctors Association, Jakarta, Indonesia |

Arun Sharma, MBA, PhD
Professor and Executive Director, JAE Leadership Institute, School of Business Administration, University of Miami, Miami, FL, USA |

Kelvin Tan, MB BCh, BSc (Hons)
Vice President Medical Affairs, Allergan Asia Pacific, Singapore
|

Kin Ping Tsang
Chair, International Alliance of Patients Organizations (IAPO), Founder, Retina Hong Kong, Past Chair, Alliance for Patients Mutual Help Organizations (APMHO), Hong Kong |

Bagus Utomo
Founder, Indonesia Community Care for Schizophrenia, Jakarta, Indonesia |

Russell Williams
President, Rx&D Canada; Chair, IFPMA Code Compliance Network, Former Member, National Assembly of Quebec, Ottawa, Canada
|
|
|
FEATURING IFPMA CODE WORKSHOP AND ETHICAL PROMOTION ROUNDTABLE
|
 The International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) is offering an IFPMA CODE WORKSHOP: HANDS-ON COMPLIANCE TRIANING on Monday, September 9, 2013 and a COMPLIANCE ROUNDTABLE: ETHICAL PROMOTION OF HEALTHCARE PRODUCTS AND THE NEED FOR A MULTI-STAKEHOLDER COLLABORATION on the morning of Tuesday, September 10, 2013.
The International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) is offering an IFPMA CODE WORKSHOP: HANDS-ON COMPLIANCE TRIANING on Monday, September 9, 2013 and a COMPLIANCE ROUNDTABLE: ETHICAL PROMOTION OF HEALTHCARE PRODUCTS AND THE NEED FOR A MULTI-STAKEHOLDER COLLABORATION on the morning of Tuesday, September 10, 2013.
Click here to register.
|
|
PLENARY SESSIONS
|
- The Role of Compliance and Ethics in the Pharmaceutical Enterprise
- Creating an Ethical Culture
- Overview of the Patient Perspective
- Overview of the Health Care Professional (HCP) Perspective
- Overview of the Distributor Perspective
- The Role of Voluntary Codes in Facilitating Compliance and Ethical Behavior
- Asia Pacific Code Roundtable
- A Local View on Anti-Corruption
- Asia Pacific Anti-Corruption Update Roundtable
- Compliance Issues relating to Patient Interactions
- Transforming the Compliance Profession & Possible Professional Certification
- Focus on Best Practices in Medical Affairs Compliance
- Best Practices in Implementing the 7 Elements of an Effective Compliance Program
- The Future of Compliance
|
|
|
|
THE FOLLOWING MINI SUMMITS
|
- Negotiating, Auditing and Monitoring Third Party Relationships
- Compliance Issues in Social Media and Digital Marketing
- Korea Compliance Update
- Japan Compliance Update
- China, Malaysia and Indochina Compliance Updates
- Compliance Best Practices
- M&A, JVs and Spinoffs Compliance Issues
- Advanced Issues in Transparency and Disclosure
- Technology-driven Monitoring Best Practices
- Privacy vs. Transparency
- Clinical Trials Compliance Issues
|
|
ASIA PACIFIC PHARMA CONGRESS IS
|

|
|
|
AND THE POSTCONFERENCE SYMPOSIUM:
COMPLIANCE AS A STRATEGIC FUNCTION BY
|

Arun Sharma, MBA, PhD
Professor and Executive Director, JAE Leadership Institute, School of Business Administration, University of Miami, Miami, FL, USA
|
|
|
GLOBAL COMPLIANCE EXPERTS
|

Jan Oliver Huber
Secretary General, Pharmig (Association of the Austrian Pharmaceutical Industry), Member, IFPMA Code Compliance Network, Vienna, Austria |

Tamara Music
Manager, Influenza Vaccines & Code Compliance, IFPMA, Geneva, Switzerland |

Chrisoula Nikidis
Director, Ethics and Compliance, Canada's Research-Based Pharmaceutical Companies (Rx&D), Ottawa, ON, Canada
|

Heather Simmonds
Director and Chair, Code of Practice Panel, Prescription Medicines Code of Practice Authority, Vice-Chair, IFPMA Code Compliance Network, London, UK |

Jose F. Zamarriego Izquierdo
Director Unidad de Supervision Deontologica, FARMAINDUSTRIA, Madrid, Spain
|
|
|
LEADING COMPANY COMPLIANCE PROFESSIONALS AND COUNSEL
|

Masood Ahmed
Vice President, Regional Compliance Officer- Asia Pacific, sanofi-aventis group, Singapore |

Alan Chin
Ethics & Compliance Officer, Malaysia & Singapore, Eli Lilly, Co-Chair, Marketing Practices Committee, Singapore Association of Pharmaceutical Industries (SAPI), Singapore |

Karen Eryou
Director and Head, Quality and Compliance, UCB Pharma - Greater China and SE Asia, Shanghai, China
|

Manuel Flores
Compliance Director Asia Pacific, Bayer (South East Asia) Pte Ltd., Singapore |

Andrew Frye, MBA
Vice President, DKSH Healthcare, Former Commercial Director, Pharma, Abbott Laboratories, Bangkok, Thailand |

Kader Garnier
Healthcare Compliance Officer for R&D, Johnson & Johnson, New Brunswick, NJ
|

Dr. Ednin Hamzah
CEO and Medical Director of Hospis Malaysia, Kuala Lumpur |

Mari Kikuchi
Compliance Director, AbbVie, Tokyo, Japan |

Yota Kikuchi
Manager, Promotion Code & Public Affairs, sanofi-aventis, Vice Chairman, Code Practices Committee, Japan Pharmaceutical Manufacturers Association (JPMA), Member, IFPMA Code Compliance Network, Tokyo, Japan
|

Jungwook Kim, Esq.
Director, Legal Operations & Business Development, GSK Korea, Seoul, South Korea |

Howard Janhong Lin
Regional Asia Operations Ethics and Compliance Officer, Lilly, Hong Kong |

Deborah Monk
Director, Compliance, Medicines Australia; Manager, Medicines Australia's Code of Conduct, Deakin, Australian Capital Territory, Australia
|

John Pane, CIPP, CIPP/IT
Regional Lead, Privacy Compliance & Data Protection - Asia Pacific, Johnson & Johnson, Former Chief Privacy Officer, Australia Post, Singapore |

Roj Rungvisai
Head of Legal and Compliance, Novartis Thailand, Chairman, Code Compliance Subcommittee, Pharmaceutical Research and Manufacturers Association (PReMA), Bangkok, Thailand |

Parulian Simanjuntak
Executive Director, International Pharmaceutical Manufacturers Group, Jakarta, Indonesia
|

Robert Skinner, PhD
Deputy Compliance Officer, Emerging Markets, Asia Pacific & Japan, GlaxoSmithKline, Chalfont St Giles, Buckinghamshire, UK |

Gonca Sönmez Uyguc, Esq.
Legal and Compliance Director, Business Area South East Asia (BAOS), Novo Nordisk Pharma Operations (BAOS) Sdn. Bhd., Kuala Lumpur, Malaysia |

Trudy Tan
Vice President Compliance - International, AstraZeneca, Shanghai, China
|

Rhys Tee
Associate Director, Compliance (APAC), Allergan, Former Associate Director, Compliance (APAC), Biogen Idec, Singapore |
Toru Terashima
Head, External Affairs, Dainippon Sumitomo Pharma Co., Ltd., Chairman, Compliance Practices Committee, Japan Pharmaceutical Manufacturers Association (JPMA), Tokyo, Japan
|

Francisco P. Tranquilino, MD, FPCP
Technical Consultant, Ethics Committee, Pharmaceutical and Healthcare Association of the Philippines (PHAP), Special Assistant to the Dean and College Secretary, University of the Philippines College of Medicine, Manila, Philippines |

Dr Vicknesh Welluppillai
Senior Medical Director, Pfizer Malaysia, Kuala Lumpur, Malaysia
|
|
|
AND TRUSTED CONSULTANTS AND COUNSEL
|

Ted Acosta, Esq.
Principal, Ernst & Young LLP, Former Senior Counsel, Office of Inspector General, US Department of Health and Human Services, New York, NY, USA and Paris, France |

Wilson Ang, Esq.
Partner, Norton Rose Fulbright (Asia) LLP, Singapore |

Wayne Baker
Senior Vice President and Chief Sales Officer, AHM, New Providence, NJ, USA
|

François Benelli
Business Compliance Consultant, Cegedim, Tokyo, Japan |

Praween Chantanakomes, Esq.
Lawyer, Baker & McKenzie, Bangkok, Thailand |

Eugene Chen, Esq.
Partner, Shanghai Office, Hogan Lovells International LLP, Shanghai, China
|

Ken Chia, Esq.
Partner, Baker & McKenzie, Wong & Leow, Singapore |

Kyung-Sun (Kyle) Choi
Kim & Chang, Seoul, Korea |
Myung Soon Chung
Kim & Chang, Seoul, Korea
|

Hwa-Soo Chung, Esq.
Chair, Health Law Group, Kim & Chang Law Firm, Seoul, South Korea |

Yee Chung Seck, Esq.
Partner, Baker & McKenzie (Vietnam) Ltd., Ho Chi Minh City, Socialist Republic of Vietnam |

Peter Claude
Partner, PricewaterhouseCoopers, San Francisco, CA, USA
|

Diva DUONG
Vice President Compliance EMEA, Cegedim, Paris, France |

Jeffrey Hessekiel, Esq.
Senior Counsel, Arnold & Porter; Former Chief Compliance & Quality Officer, Gilead Sciences, Inc., San Francisco, CA, USA |

Erinn Hutchinson
Partner, Advisory Services, PricewaterhouseCoopers Consultants (Shenzhen) Limited, Shanghai, China
|

Jack Jia
Senior Manager, Ernst & Young, Hong Kong |

Rajiv Joshi
Director, Assurance, Ernst & Young LLP, Mumbai, India |

Elizabeth H. Kim, Esq.
Manager, Regulatory and Compliance Services, Porzio Life Sciences; Associate, Porzio, Bromberg & Newman, Morristown, NJ, USA
|

Masaru Kitamura, JD, LLM
Founder, KitamuraLaw, Tokyo, Japan |

Lei Li, LLB, LLM
Of Counsel, Sidley Austin LLP, Former Attorney, Ministry of Commerce (MOFCOM) of the Government of the People's Republic of China, Beijing, China |

David McKeering
Partner and National Health Practice Leader, Advisory Services, PricewaterhouseCoopers, Brisbane, Australia
|

Kirk Ogrosky, Esq.
Partner, Arnold & Porter, Former Head of Healthcare Fraud Enforcement, Criminal Division, US Department of Justice, Washington, DC, USA |

Rajesh Sreenivasan, Esq.
Partner, Rajah & Tann, Singapore |

Kareena Teh
Partner, Baker & McKenzie, Hong Kong
|

Yuet-Ming Tham, Esq.
Partner, Sidley Austin LLP, Hong Kong |

Mai Tran
Executive Vice President of Health & Wellness, Ruder Finn Asia, Singapore |

Kherk Ying Chew, Esq.
Managing Partner, Wong & Partners , Kuala Lumpur, Malaysia
|
|
THIRD ASIA PACIFIC PHARMACEUTICAL COMPLIANCE CONGRESS AND BEST PRACTICES FORUM
September 10 - 12, 2013
|
ATTEND ONSITE
PARKROYAL Kuala Lumpur Hotel
Kuala Lumpur, Malaysia
|
OR |
WEBCAST PARTICIPATION
In your own office or home live via the Internet with 24/7 access for six months
|
 |
|

|
|

|
UPDATED AGENDA
AVAILABLE HERE
|
Click here for agenda.
|
|
|
|
IFPMA FLYER NOW AVAILABLE
|
Click here to download the IFPMA flyer.
|
|
|
|
IFPMA SEPTEMBER 10 PROGRAM
|
Click here to download the IFPMA September 10, 2013 Program.
|
|
|
|
SPONSORED BY:
|
|

The Asia Pacific Healthcare Industry Compliance Team is an ad hoc, voluntary group of Asia Pacific pharmaceutical, medical device and other life sciences company compliance professionals and legal counsel who meet quarterly to discuss legal and compliance issues and best practices.
|
|
COSPONSORED BY:
|
|

The International Society of Healthcare Ethics and Compliance Professionals (ETHICS) is a forum for open dialogue and exchange among Compliance and Ethics professionals in the Healthcare industry. Association's activities are developed around the four following pillars, sustained by one transverse and permanent objective: support professionals in Compliance and Ethics in Healthcare industry to develop and better manage their role and duties -- without any sort of commercial benefit for the Association or any of its members.

The Pharmaceutical Compliance Forum (PCF) is a coalition of senior compliance professionals and legal counsel from more than 50 of the largest research-based pharmaceutical manufacturers. The PCF was founded in early-1999 by compliance professionals from the pharmaceutical industry to promote effective corporate compliance programs.
|
|
|

|
|
CO CHAIRS
|

Gareth Lee, Esq.
General Counsel & Head of Compliance, Asia Pacific, Allergan Inc., Singapore
|

Abdul Luheshi, MBA
Vice President Health Care Compliance, Asia Pacific, Johnson & Johnson, Singapore
|

Maria "Maru" Quindimil, MBA
Executive Director, Regional Compliance Officer, Asia Pacific and India, Merck Sharp and Dhome (Asia Ltd.), Manila, Philippines
|

Tom M. Zerull
Regional Compliance Director, Asia, Australia, Africa, Middle East & Japan, AbbVie, Former Regional Director, Asia Pacific, Office of Ethics & Compliance, Abbott, Singapore
|
|
GRANTORS:
|
|
DIAMOND
|

|
|
PLATINUM
|

|
|
BRONZE
|







|
|
2013 ASIA PACIFIC PHARMA CONGRESS PLANNING COMMITTEE
|
Gareth Lee, Esq.
General Counsel & Head of Compliance, Asia Pacific, Allergan Inc., Singapore (Co chair)
Abdul Luheshi, MBA
Vice President Health Care Compliance, Asia Pacific, Johnson & Johnson, Singapore (Co chair)
Maria "Maru" Quindimil, MBA
Executive Director, Regional Compliance Officer, Asia Pacific and India, Merck Sharp and Dhome (Asia Ltd.), Manila, Philippines (Co chair)
Tom M. Zerull
Regional Compliance Director, AAAME & Japan, AbbVie, Former Regional Director, Asia Pacific, Office of Ethics & Compliance, Abbott, Singapore (Co chair)
Masood Ahmed
Vice President, Regional Compliance Officer- Asia Pacific, sanofi-aventis group, Singapore
John Auerbach, MA
Partner, Fraud Investigation & Dispute Services, Ernst & Young, Shanghai, China
Wayne Baker
Senior Vice President and Chief Sales Officer, AHM, New Providence, NJ, USA
Scott Bass, Esq.
Partner and Chair, Global Life Sciences Team, Sidley Austin LLP, Washington, DC, USA
François Benelli
Business Compliance Consultant, Cegedim, Tokyo, Japan
Jeff Berry
Regional Director, Ethics and Compliance, Asia Pacific & Japan, Abbott, Singapore
Maija Burtmanis, Esq.
Associate General Counsel, Alcon Asia, Singapore
Eugene Chen, Esq.
Partner, Shanghai Office, Hogan Lovells International LLP, Shanghai, China
Bernice Cheng, Esq.
Executive Counsel, Transactions Compliance, Asia Pacific, GE Corporate, Shanghai, China
Peter Chua
Compliance Director, Asia Pacific, Covidien, Singapore
Hwa-Soo Chung, Esq.
Chair, Health Law Group, Kim & Chang Law Firm, Seoul, South Korea
Sue Egan
Director and Principal Consultant, Sue Egan Associates, Editor, Life Science Compliance, Buckinghamshire, UK
Marc Eigner, MS, MBA
Partner, Polaris, New York, NY, USA
Karen Eryou
Director and Head, Quality and Compliance, UCB Pharma - Greater China and SE Asia, Shanghai, China
Manuel Flores
Compliance Director Asia Pacific, Bayer (South East Asia) Pte Ltd., Singapore
Erinn Hutchinson
Partner, Global Pharmaceuticals and Life Sciences, PwC, Shanghai, China
Daniel A. Kracov, Esq.
Partner and Head, FDA and Healthcare Practice, Arnold & Porter, Washington, DC, USA
Howard Janhong Lin
Regional Asia Operations Ethics and Compliance Officer, Lilly, Hong Kong
Liz MacGillivray
Compliance Director, Corporate Integrity and Compliance, Novartis International AG, Basel, Switzerland
Tamara Music
Manager, Influenza Vaccines & Code Compliance, IFPMA, Geneva, Switzerland
Michael O'Connor, MS
Executive Director, IS Business Consulting, Boehringer Ingelheim, New York, NY, USA
John Patrick Oroho, Esq.
Executive Vice President and Chief Strategy Office, Porzio Life Sciences, LLC, Morristown, NJ, USA
Dusan Rehak, Esq.
Regional Compliance Director - Asia, Pfizer, Kuala Lumpur, Malaysia
Gonca Sönmez, Esq.
Compliance Counsel, Novo Nordisk Region International Operations, Zürich, Switzerland
Sabina Sudan
Vice President, Compliance Officer - Emerging Markets Asia Pacific (EMAP), Japan & Europe Pharma, GlaxoSmithKline Pte Ltd., Singapore
Trudy Tan
Vice President Compliance - International, AstraZeneca, Shanghai, China
Rhys Tee
Associate Director, Asia Pacific, Allergan Singapore Pte Ltd, Singapore
Peerapan Tungsuwan, LLM
Partner and Chair, Asia Pacific Life Sciences Group, Baker & McKenzie, Bangkok, Thailand
Douglas Worthington, Esq.
Head of Compliance & Ethics, Asia Pacific, Bristol-Myers Squibb, New York, NY, USA
Kevin Yamaga-Karns, Esq.
Senior Director, Legal and Compliance, Eli Lilly Japan, Hyogo, Japan
|
|
OTHER INTERNATIONAL PHARMA CONGRESSES
|

2007 - Brussels, Belgium

2008 - Paris, France

2009 - Rome, Italy

2010 - Berlin, Germany

2011 - Istanbul, Turkey

2011 - Singapore

2012 - Budapest, Hungary

2012 - Shanghai, China

2012 - São Paulo, Brazil

2013 - Madrid, Spain

2013 - Kuala Lumpur, Malaysia
|
|
|
FOLLOW ASIA PACIFIC PHARMA CONGRESS ON
|

|

|
|

|



