
|

|
FOURTH ASIA PACIFIC
PHARMACEUTICAL
COMPLIANCE CONGRESS
AND BEST PRACTICES
FORUM
|
ATTEND ONSITE
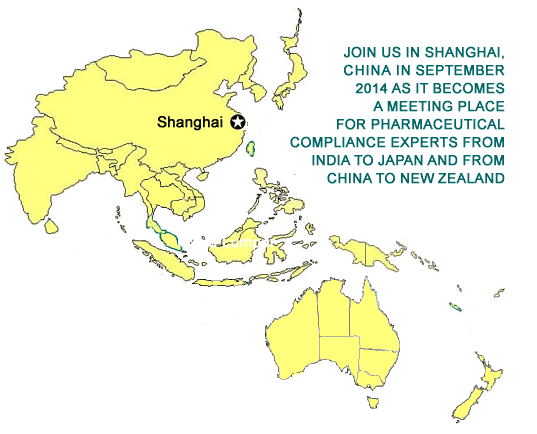
September 16 - 18, 2014
Intercontinental Shanghai
Pudong Hotel
Shanghai, China |
WEBCAST PARTICIPATION
In your own office or home live via the Internet with 24/7 access for six months
|

|
KEYNOTE SPEAKERS
|

Abdul Luheshi, MBA
Vice President, Health Care Compliance, Johnson & Johnson, Former Co-chair, Asia Pacific Pharma Congress, London, UK |

David Snow, MBA
President, China and Hong Kong, AstraZeneca, Shanghai, China |

Veronique Toully, MBA, DVM
Managing Director, China, UCB, Shanghai, China
|

Kin Ping Tsang
Chair, International Alliance of Patients Organizations (IAPO), Founder, Retina Hong Kong, Past Chair, Alliance for Patients Mutual Help Organizations (APMHO), Hong Kong |

Professor Huang Yong
Competition Law Center, University of International Business and Economics, Executive Vice Chair, Expert Consultation Group, Anti-Monopoly Committee, State Council, Peoples Republic of China, Beijing, China |

Shirley Zhao, MBA
President, Allergan China, Shanghai, China
|
|
|
FEATURING PRECONFERENCE
|
- Negotiating, Implementing, Managing, Auditing and Monitoring Third Party Relationships
|
|
PLENARY SESSIONS
|
- Asia Pacific Pharma Congress Vision and Overview
- Perspectives from the C-Suite: Ethical Business Practice
- Asia Pacific Compliance Issues from a Global Perspective
- Asia Pacific Stakeholders Roundtable: HCPs, Hospitals and Patients
- Key Issues Overview/Mini Summit Preview
- China's Health System and How it is Being Reformed
- The Chinese Anti-unfair Competition Law
- Overview of Recent Chinese Laws and Regulations
- Chinese Compliance Best Practices Roundtable
- Enhancing the Professional Role of a Compliance Officer
- Asia Pacific R&D and Clinical Trials Compliance
- Third Party Relationships Roundtable
- New Pharma Business Models
- New Compliance Challenges
|
|
|
|
AND MINI SUMMITS
|
- Training on Compliance and Ethics Basics: Program Basics
- Hot Asia Pac Compliance Issues
- Country Compliance Updates: Malaysia and Indochina
- Building the Compliance Profession
- Advanced Issues in Anticorruption Compliance
- Training on Compliance and Ethics Basics: Core Compliance Risks
- Country Compliance Updates: Myanmar, India and Indonesia
- Investigations Updates
- Public Procurement, Tendering, Reimbursement and Pricing Update
- The Role of Forensics in Compliance
- Training on Compliance and Ethics Basics: Evolving Issues, Rising Expectations
- Compliance Best Practices
- Country Compliance Updates: Korea, Japan and Taiwan
- Social Media and Technology Tools for Compliance
|
|
ASIA PACIFIC PHARMA CONGRESS IS
|

|
|
|
|
FEATURED FACULTY
|

Ted Acosta, Esq.
Principal, Ernst & Young LLP, Former Senior Counsel, Office of Inspector General, US Department of Health and Human Services, New York, NY, USA and Paris, France |

Masood Ahmed
Vice President, Regional Compliance Officer Asia & JPAC, Sanofi Group, Singapore |

Timothy Ayers, JD, MPH
Principal, Porzio, Bromberg & Newman, Morristown, NJ, USA
|

Cheryl Bai, Esq.
Associate General Counsel, Greater China, Allergan Inc., Shanghai, China |

Dane Chamorro
Managing Director, Control Risks South-east Asia, Singapore |

Chi Chen
EY Associate Director FTDS, FIDS, Shanghai, China
|

Eugene Chen, Esq.
Partner, Shanghai Office, Hogan Lovells International LLP, Shanghai, China |

Iona Cheng
Practice Leader, Integrity Risk Consulting, Greater China and North Asia, Shanghai, China |

Kherk Ying Chew, Esq.
Managing Partner, Wong & Partners, An affiliate of Baker & McKenzie, Kuala Lumpur, Malaysia
|

Chester Chu, CPA, CFE, CGMA
Director, Fraud Investigation & Dispute Services, EY, Taipei, Taiwan |

Hwa-Soo Chung, Esq.
Chair, Health Law Group, Kim & Chang Law Firm, Seoul, South Korea |
Myung Soon Chung
Foreign Attorney, Health Practice Group, Kim & Chang, Seoul, Korea
|

Yee Chung Seck, Esq.
Partner, Baker & McKenzie (Vietnam) Ltd., Ho Chi Minh City, Socialist Republic of Vietnam |

Sangeet Dalliwall, LLB, LLM
Director of Compliance, APAC, Carlson Wagonlit Travel, Singapore |

Damjan P. DeNoble, JD, MA
Partner, Rubicon Strategy Group, Editor, Health Intel Asia, Seattle, WA, USA
|

Sanjay Dhawan
Pharma GRC Leader; Executive Director, Risk Advisory Services, PwC, Bangalore, India |

Carole Ducrest, Esq.
Regional General Counsel, APAC, China, Japan, Merck KGaA, Singapore |

Lars Eckerlein
Partner, Fraud Investigation & Dispute Services, Ernst & Young, Beijing, China
|

Benjamin Ee
Associate Director, PwC, Singapore |

Adrian Emch, LLM
Partner, Hogan Lovells International LLP, Beijing, China |

Cahyani Endahayu
Associate Partner, Hadiputranto, Hadinoto & Partners, An affiliate of Baker & McKenzie, Jakarta, Indonesia
|

James Finch, Esq.
Partner and Head of Myanmar Office, DFDL/Myanmar Thanlwin Legal Services Ltd., Yangon, Myanmar |

Steven N. Gersten, Esq.
Vice President, Ethics & Compliance Officer, International and Global Marketing, AbbVie, Former Divisional Vice President and Associate General Counsel, Commercial Legal Operations, Abbott Laboratories, Chicago, IL, USA |

Dario Ghoddousi
Senior Vice President of Global Transparency and Master Data Management Solutions, Cegedim Relationship Management, Paris, France
|

Michelle Y.L. Gon, Esq.
Partner, Baker & McKenzie, Shanghai |

Erinn Hutchinson
Partner, Advisory Services, PricewaterhouseCoopers Consultants (Shenzhen) Limited, Shanghai, China |

Sandy S. Johnston
Partner, PwC, Shanghai, China
|

Jonathan Kellerman
Principal, Pharmaceutical and Life Sciences Advisory, PwC, Florham park, NJ |

Kitchrat Kontain, LLM, MBA
Managing Partner, South East Asia Law Office, Vientiane, Laos |

Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC, USA
|

Cristopher Landrito, LIB
Regional Compliance Officer, APAC Group Compliance, Merck Pte. Ltd., Singapore |
Yunjoh Lee
Attorney, Health Practice Group, Kim & Chang, Seoul, Korea |

Ren Jun Lim
Partner, Baker & Mckenzie, Singapore
|

Lei Li, LLB, LLM
Partner, Sidley Austin LLP, Beijing, China |

Steffanie Lim-Ho
Head of Ethics & Compliance, Lilly China, Shanghai, China |

Chia-Feng Lu, Esq.
Associate, Baker & McKenzie (Gaikokuho Joint Enterprise), Tokyo, Japan
|

Andrew Martin, Esq.
Principal, Baker & McKenzie, Wong & Leow, Singapore |

Shawn McBride
Vice President & General Manager - Direct to Patient and Market Access, Cardinal Health, Shanghai, China |

Ramesh Moosa
Partner, Forensic Technology Solutions, PwC, Shanghai, China
|

Dinesh Moudgil
Executive Director, EY, Mumbai, Maharashtra, India |

Arthur Muratyan, Esq.
Secretary General, International Society of HealthCare Ethics and Compliance Professionals (ETHICS), Paris, France |

Clement Ngai, Esq.
Partner, Baker & McKenzie Shanghai Office, Shanghai, China
|

Sun Qiang
Deputy Dean, Assistant Professor, Center for Health Management and Policy, School of Public Health, Shandong University, Jinan, China |
Teng Ren, MA
Head of Compliance, Asia-Pacific, Middle East, and Africa, Russia/Ukraine, Novartis, Former Compliance Director, Johnson & Johnson Medical China and Eli Lilly, Singapore |

Bretton G. Sciaroni, Esq.
Partner, Sciaroni & Associates, Phnom Penh, Cambodia
|

Clarissa SHEN, MBA
Country Compliance Officer, Global Compliance and Business Ethics, Sanofi, Shanghai, China |

Benjamin Shobert, MBA
Partner and Founder, Rubicon Strategy Group, Seattle, WA |

Yuthana Sivaraks
Partner, Baker & McKenzie Ltd., Bangkok, Thailand
|

John Tan, Esq.
Counsel, Reed Smith, Former China Regional Compliance Director, Pfizer, Shanghai, China |

Julie Tan
Senior Compliance Manager, AstraZeneca, Shanghai, China |

Rhys Tee
Associate Director, Compliance (APAC), Allergan, Singapore
|

John Tsai
Partner, Fraud Investigation & Dispute Services, Ernst & Young, Shanghai, China |

Emmanuel Vignal
Partner, Fraud Investigation & Dispute Services, Ernst & Young, Shanghai, China |

Kevin Y. H. Wang, LLM
Senior Consultant, Baker & McKenzie, Taipei, Taipei, Taiwan
|

Wendy L. Wysong, Esq.
Partner, Clifford Chance LLP, Former Assistant US Attorney, Washington, DC, Former Deputy Assistant Secretary for Export Enforcement, Bureau of Industry & Security, UA Department of Commerce, Hong Kong and Washington, DC, USA |

Chen Yang, Esq.
Partner and Head, China Life Sciences Practice, Sidley Austin LLP, Former Legislative Official, Bureau of Legislative Affairs, Chinese State Council, Beijing, China |

Shine Yao, MIM
China Compliance Director, Merck Sharp & Dohme, Shanghai, China
|

Eric Young
Forensic Technology Partner, EY, Hong Kong |

Bing Yu, LLB, LLM
Of Counsel, Clifford Chance LLP, Shanghai, China |

Richard Yun
Partner, King & Wood Malleson, Beijing, China
|

Marx Zhou
Baxter (china Investment Co., Ltd), Sr. Compliance Manager/ Asia Pacific, Shanghai, China |

Stella Zhu, LLM
Senior Counsel, Asia Pacific for R&D, Allergan, Shanghai, China
|
|
|

|
|
SPONSORED BY:
|
|

The Asia Pacific Healthcare Industry Compliance Team is an ad hoc, voluntary group of Asia Pacific pharmaceutical, medical device and other life sciences company compliance professionals and legal counsel who meet quarterly to discuss legal and compliance issues and best practices.
|
|
COSPONSORED BY:
|
|

The International Society of Healthcare Ethics and Compliance Professionals (ETHICS) is a forum for open dialogue and exchange among Compliance and Ethics professionals in the Healthcare industry. Association's activities are developed around the four following pillars, sustained by one transverse and permanent objective: support professionals in Compliance and Ethics in Healthcare industry to develop and better manage their role and duties -- without any sort of commercial benefit for the Association or any of its members.

The Pharmaceutical Compliance Forum (PCF) is a coalition of senior compliance professionals and legal counsel from more than 50 of the largest research-based pharmaceutical manufacturers. The PCF was founded in early-1999 by compliance professionals from the pharmaceutical industry to promote effective corporate compliance programs.
|
|
|

|
|
CO CHAIRS
|

Maija Burtmanis, LLB, LLM
Regional Compliance Director, Asia, Australia, Africa, Middle East & Japan, AbbVie, Singapore
|

Karen Eryou
Senior Director, Corporate Compliance APAC, UCB Pharma, Shanghai, China
|

Gareth Lee, Esq.
General Counsel and Head of Compliance, Asia Pacific, Allergan Inc., Singapore
|

Maria "Maru" Quindimil, MBA
Executive Director, Regional Compliance Officer, Asia Pacific and India, Merck Sharp and Dhome (Asia Ltd.), Manila, Philippines
|
|
CONTINUING EDUCATION CREDITS
|
- Accounting Professionals: Approved for up to 17.0 NASBA CPE credits.
- Compliance Professionals: Approved for up to 22.2 Compliance Certification Board CCB Credits.
- Attorneys:
- Approved to offer a maximum of 17.00 Hours of California CLE Credit.
- Approved to offer a maximum of 15.50 Hours of Pennsylvania MCLE Credit.
Click here for more information.
|
|
GRANTORS:
|
|
DIAMOND
|

|
|
PLATINUM
|

|
|
BRONZE
|







|
|
MEDIA PARTNER
|

|
|
OTHER INTERNATIONAL PHARMA CONGRESSES
|

2007 - Brussels, Belgium

2008 - Paris, France

2009 - Rome, Italy

2010 - Berlin, Germany

2011 - Istanbul, Turkey

2011 - Singapore

2012 - Budapest, Hungary

2012 - Shanghai, China

2012 - São Paulo, Brazil

2013 - Madrid, Spain

2013 - Kuala Lumpur, Malaysia

2014 - Dubai, United Arab Emirates

2014 - Mexico City, Mexico
|
|
|

|



